22+ nernst equation calculator
Tutor Pace offer students help with Nernst Equation Calculator for any grade in any subject including math algebra trigonometry and geometry. T Temperature.
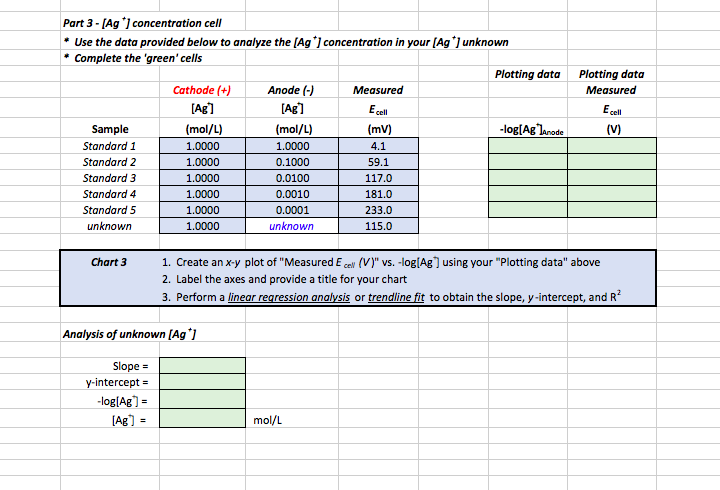
Part 1 Nernst Equation Calculator Cell Potentials Chegg Com
Standard cell potentials are calculated in standard conditions of temperature and pressure.

. The Nernst equation for 298 Kelvin can be represented as follows. The Nernst equation can be used to calculate. The Nernst Equation 14 of 22 me remst equation is one of the most important equations in electrochemistry.
E E0 00592n log_10 Q Hence as per the Nernst equation the potential of the electrochemical cell depends on the. E 0021544 T ln p O 2 p O 2 And using log10. This calculator will allow the calculation of the expected.
However in non-standard conditions the Nernst equation is used to. View Presentation9-Notes-NernstEquationpdf from CHEM 178 at Iowa State University. The following is the procedure how to use the Nernst equation calculator Step 1.
K c 322 10 30. The following example shows how the Nernst equation may be used to calculate the potential of an electrochemical cell at non-standard conditions. In the input field enter the standard half-cell reduction potential chemical activity for the reductant and oxidant.
The Nernst equation which measures electrical energy is used to find the cell potential or voltage of a system. The following formula is used to calculate the reduction potential of a cell reaction. To calculate the cell potential at non-standard-state conditions.
E o Standard Electrode potential of the cell. Electric Potential Calculator. CHEM 178 Chemical Thermodynamics Lecture Presentation 9.
22 Nernst Equation 1 How can we. N The number of electrons. E E0 RTZf ln Q.
The emf and the standard emf of a cell in the following reaction is 5V and 506V at room temperature Ni s. E -00496 T log 10 pO. E cell Electromotive force of the cell.
The Nernst equation calculates the equilibrium potential also referred to as the Nernst potential for an ion based on the charge on the ion ie its valence and its. The formula was developed by Nobel Prize winner Walther Nernst and is. E 00496 T log 10 p O 2 p O 2 The output of both equations is in mV.
The equation may be re-arranged to allow calculation of the emf from a known reference concentration and a measured oxygen concentration.

Worked Example Nernst Equaiton Youtube
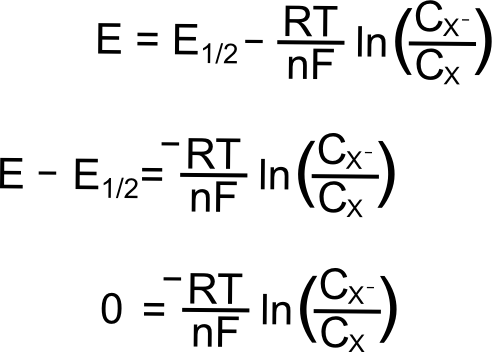
The Nernst Equation
Nernst Equation Calculator Calistry

Neuroscience Nernst Equation Biology Stack Exchange
Why Is The Quantity Inside The Logarithm In The Nernst Equation Not Unitless Quora

Neet Chemistry Notes Electrochemistry Nernst Equation Cbse Tuts
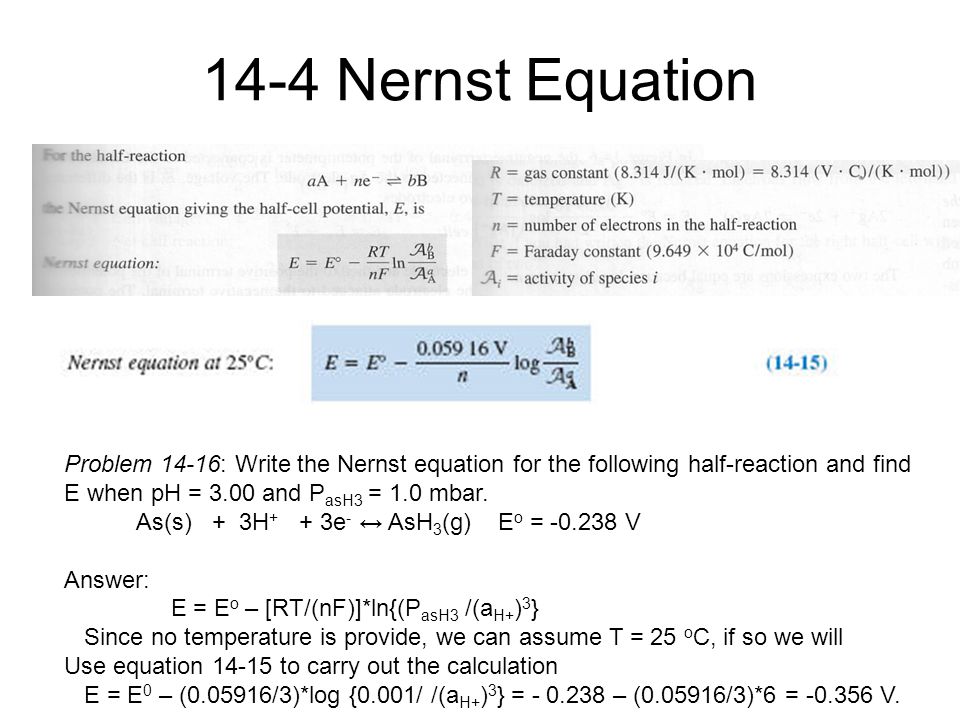
Chemistry Analytical Chemistry Fall Ppt Video Online Download

Calculating Cell Potentials In Nonstandard Conditions Chemistry Study Com
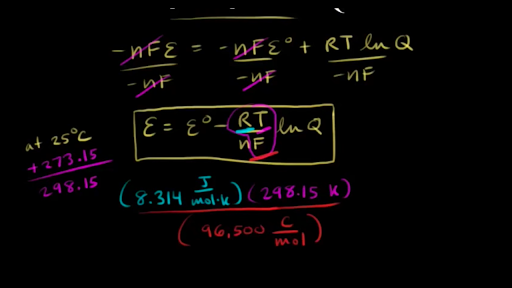
Nernst Equation Video Electrochemistry Khan Academy

20 6 The Nernst Equation Chemistry Libretexts

Nernst Equation Osmosis

Nernst Equation Calculator
How To Calculate And Solve For Nernst Equation Corrosion Nickzom Blog
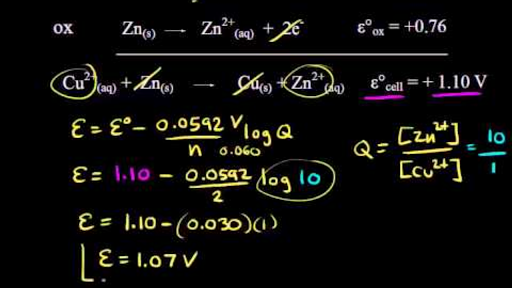
Using The Nernst Equation Video Khan Academy

Write The Nernst Equation And Emf Of The Following Cells At 298 K I Mg S Mg 2 0 001 M Cu 2 0 0001 M Cu S Ii Fe S Fe 2 0 001 M H 1m H2 G 1bar Pt S Iii

Nernst Potential Calculator Physiologyweb
:max_bytes(150000):strip_icc()/illustration-of-daniell-cell--electrochemical-cell-consisting-of-copper-and-zinc-plates-immersed-in--96168851-5b7eec1346e0fb0050f8ba15.jpg)
Nernst Equation Calculate Cell Potential